%%{init: {'theme': 'base', 'themeVariables': { 'primaryColor': '#f0f0f0', 'edgeLabelBackground':'#ffffff', 'tertiaryColor': '#f0f0f0'}}}%%
flowchart TD
A[Start Application] --> B[Select Section 21 Application]
B --> C[Select Medicine Category]
C --> D[Choose Patient Type]
D --> E{Patient Type?}
E -->|Named Patient| F[Complete Named Patient Form]
E -->|Multiple Patient| G[Complete Multiple Patient Form]
F --> H[Fill Applicant Details]
G --> H
H --> I[Fill Importer Information]
I --> J[Fill Medicine Details]
J --> K[Process Payment]
K --> L[Application Submitted]
Overview
What are Section 21 Applications?
Section 21 of the Medicines and Related Substances Act allows for the use of unregistered medicines in South Africa under specific circumstances. The Section 21 application process enables healthcare professionals to request authorization from SAHPRA to access and use medicines that are not registered in South Africa
Types of Section 21 Applications
There are two main types of Section 21 applications:
- Named Patient Applications: For individual patients requiring unregistered medicine
- Multiple Patient Applications: For groups of patients requiring the same unregistered medicine

Application Process Overview
Key Information Required
Regardless of the application type, you will need to provide the following:
Select below to view and download a checklist for your Section 21 Application
1. Section A: Particulars of the Applicant
Note: For this section all fields are required.
- Title (Dropdown) - Required
- Full Name and Initials (Text input) - Required
- Surname (Text input) - Required
- Health Professions Council (South Africa) Registration Number (Text input) - Required
- HPCSA registration certificate (File upload) - Required
- Registered Qualifications (Text input) - Required
- Registered specialty under which you are currently practising and treating the patient mentioned in section C below (Text Field) - Required
- Practice Number (BHF No) (Text input) - Required
- Cellular Phone Number (Text input) - Required
- Site Address (Searchable input) - 1 Required
- Add Address (Optional additional address)
- Contact Person(s) to answer queries/outcome about the unregistered medicine - 1 Required
- Cellular Phone Number (Text input)
- E-mail address (Text input)
- Add Contact (Optional additional contact)
2. Section B: Licensed Importer and Distributor Details
Note: For this section the dropdown values can be used or text values can be entered but the fields for Licensed Importer are required for a valid outcome report.
- Select Licensed Importer (Dropdown with searchable list) - Required
- Status (read-only field)
- SAHPRA License Number (Text input or auto-filled)
- SMF Number (Text input or auto-filled)
- Physical Site Address (Searchable field)
- Select Licensed Distributor - Not Required
- Select Name (Dropdown with searchable list)
- Status (Auto-filled or read-only field)
- SAHPRA License Number (Text input or auto-filled)
- SMF Number (Text input or auto-filled)
- Physical Site Address (Searchable input)
3. Section C: Particulars of the Patient
Note: For this section the fields are applicable to a Named Patient Application. Only the Diagnosis is applicable for a Multi-Patient application.
- Title (Dropdown) - Required
- First Name (Text input) - Required
- Surname (Text input) - Required
- Age (Text input or number field) - Required
- Gender (Dropdown) - Required
- Weight (Kg) (Text input) - Required
- Height (Cm) (Text input) - Required
- Residential Address (Text input) - Required
- Occupation (Text input) - Required
- Telephone Number (Office Hours) (Text input) - Required
- Cellular Number (Text input) - Required
- Positive Proof of Identification (File upload) - Non Required
- Diagnosis (ICD-11 Code) (Searchable dropdown)
- Severity (Dropdown) - Required
- Staging (Dropdown) - Required
- Prognosis (Dropdown) - Required
- Treatment Regimen (Dropdown) - Required
- Details of Current Treatment Regimen (Text input) - Required
- Add Diagnosis and treatment (Optional button to add additional entries)
- Concomitant Disease/s - Required (Brief description including severity, staging, prognosis where applicable. Indicate the current standard treatment regimen for the concomitant disease.)
- Diagnosis (ICD-11 Code) (Searchable dropdown) - Required
- Severity (Dropdown) - Required
- Staging (Dropdown) - Required
- Prognosis (Dropdown)- Required
- Treatment Regimen (Dropdown) - Required
- Details of Current Treatment Regimen (Text input) - Required
- Informed Consent of Patient (File upload) - Required
4. Section D: Particulars of the Unregistered Medicine
1. API (Active Pharmaceutical Ingredient) Details search
- INN Name (Auto-filled or editable if manual override enabled after search) - Required
- Manufacturer (Auto-filled or editable if manual override enabled after search) - Not Required
- API Manufacturer Number Type (Auto-filled or editable if manual override enabled after search.) - Not Required
- Number (Auto-filled or editable if manual override enabled after search) - Not Required
2. Final Product Manufacturer Information
- Manufacturer Search by Name (Search field) - Required
- SMF Number (Text input) - Not Required
- GMP Information
- Approval Number (Text input) - Not Required
- Approval Date (Date picker) - Not Required
- Name of Regulatory Body that Issued Certificate (Text input) - Not Required
- Upload GMP Certificate (File upload) - Not Required
3. Medicine Formulation, Quantity, Prescription, and Treatment Plan Details
- Proposed Proprietary Name (Text input) - Required
- Strength (Numeric input) - Required
- Unit of Measurement (Dropdown e.g., mg, ml, IU) - Required
- Route of Administration (Dropdown e.g., Oral, IV) - Required
- Dosage Form (Dropdown e.g., Tablet, Injection) - Required
- Pack Size (Numeric input) - Required
- Intended Patient Pack Selling Price (ZAR) (Currency input) - Required
- Upload Medical Prescription (File upload) - Required
- Upload PI/PIL Information (File upload) - Required
- Dosage Unit (e.g., 1 tablet) (Text input) - Required
- Dosage Interval (e.g., Every 8 hours) (Text input) - Required
- Duration of Total Treatment (e.g., 7 days) (Text input) - Required
4. Registration Status in Other Countries
- Registered / Unregistered (Radio buttons) - Not Required
- Select Country (Dropdown) - Not Required
- In which country is the Patient Pack Registered (Text input) - Not Required
- NRA Name (Text input) - Not Required
- Name of National Regulatory Authority (Text input) - Not Required
5. SAHPRA-Registered Medicines for the Unmet Medical Need
- Search SAHPRA Medicines Registry (Search field) - Required
- Reason for Not Using a SAHPRA-Registered Medicine or Treatment Regimen (Text area) - Required
- Upload Supporting Document for Reason (File upload) - Not Required
5. Supporting Documentation
Below is the list of possible additional documents required:
- Manufacturing license
- GMP Certificate
- Approved or proposed Product package insert
- Motivation letter
- Linked to dosing evidence from peer-reviewed articles
- Peer reviewed articles for the use in the stated diagnosis, copy of publication in peer reviewed scientific journal
- MC & S reports
- Out-of-stock letter
- Discontinuation letter
- Cultivation Licence
- Certificate of Analysis (COA), Professional Information
- Patient information leaflet
- Target Product Profile
- Investigational Medicinal Product Brochure
- Clinical Study Reports
- Clinical Development Plan
- Meta-analyses
- Risk Management Plan
- Public assessment reports
- Country-specific documents
- Access Program pre-approval form
6. Document Upload Requirement
- Please rename any uploaded files to contain the application number for the application you are currently completing
- eg. “GPM_Certificate_S2100000010.pdf”
7. Accessing your Signed Outcome Letter
- Once your application has been rejected or approved the Signed Outcome Letter will reside in the Application Documents section of the application. Simply find the application on your Application Manager and click on the Overview option under the Actions available on the right-hand side of the application.
Fees and Payment
- Note: The application fee will be waived for applicants who reside in a Public Healthcare Institution registered as such on the portal.
- Applicants must first complete the whole application of which at the end an order number will be generated. This generated order number must be used as the beneficiary reference number on the proof of payment.
- To initiate a refund, refer to the SAHPRA Payment Guideline
Submitting a Revised Proof of Payment Following a Disputed Transaction
After receiving an email stating that your payment was disputed for your application, you can submit a new proof of payment for that application.
Navigate to the Applications Dashboard
Locate your application you wish to submit a new proof of payment for
Click on the Action button to the right of the application
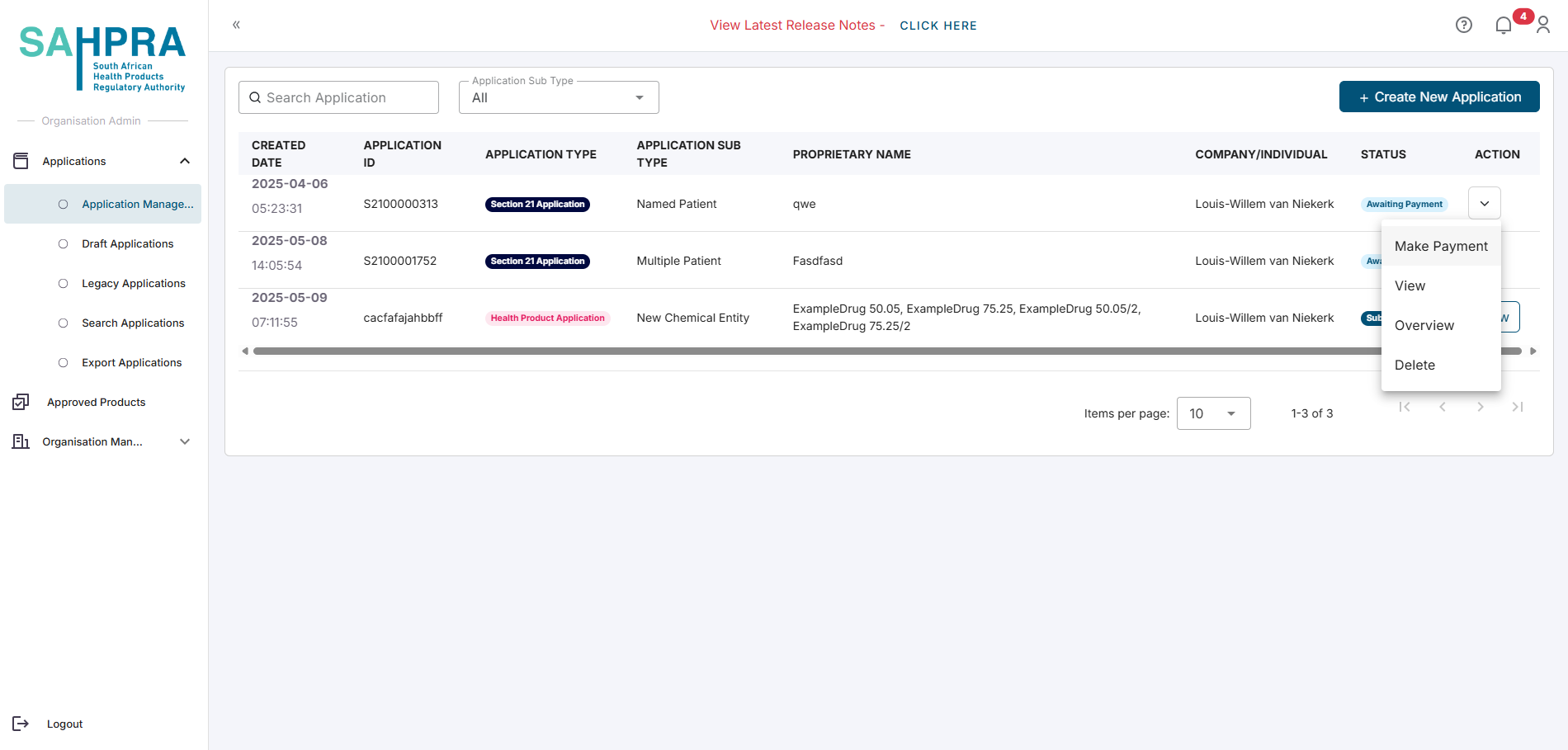
Select Make Payment
Upload and Submit your new proof of payment
Section 21 applications require payment of the prescribed fee. The payment process is similar to other SAHPRA applications:
- Submit the application
- View the order details
- Make payment via EFT
- Upload proof of payment
- Wait for payment verification
Processing Timeframes
- The processing for all applications will take up to three working days (Excluding the timeframe in which applications are Queried)
- Applications are deemed submitted once the payment has been approved by SAHPRA
Next Steps
- For Named Patient applications, proceed to Named Patient Applications
- For Multiple Patient applications, proceed to Multiple Patient Applications
